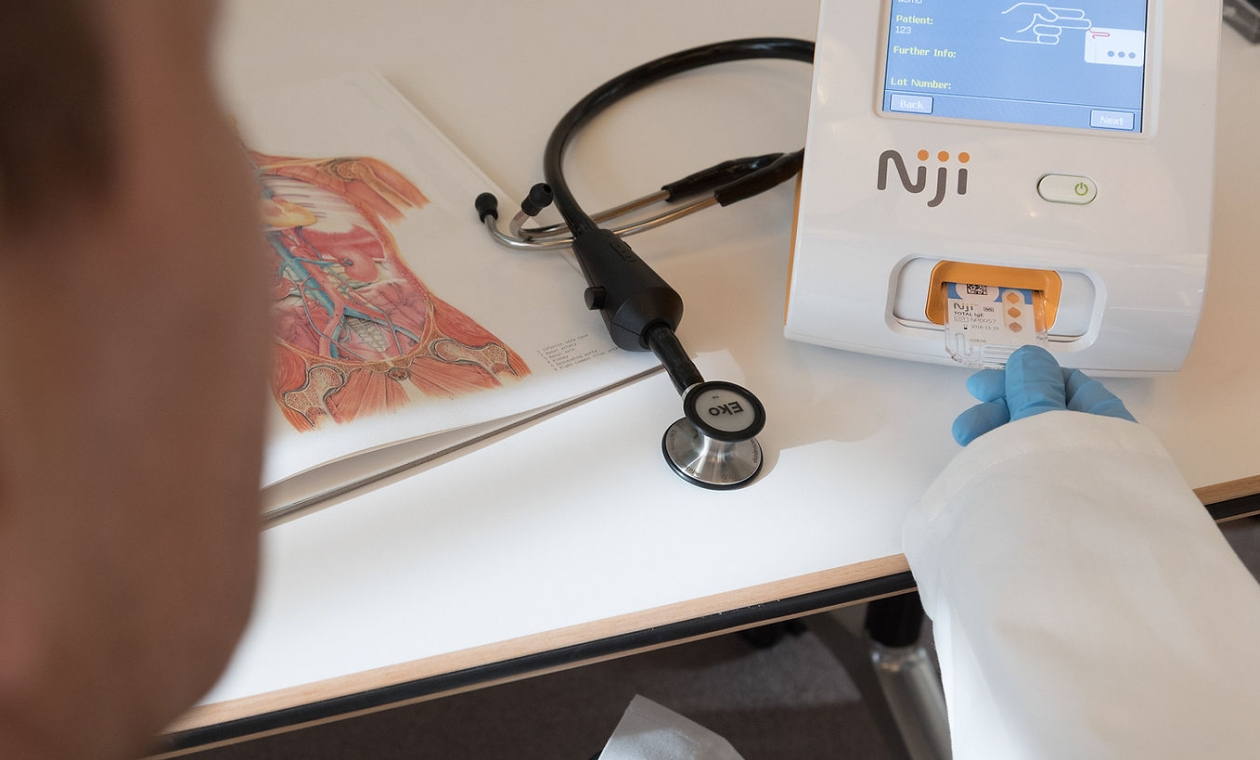
Point of care device
THE primary objective was to create a rapid and reliable point of care system for diagnosing thyroid dysfunction using blood. In order to achieve this critical goal, Maddison was chosen as the key development partner. Together, we collaborated on designing both the reader and disposable microfluidic cartridge. Utilizing Vivacta's cutting-edge piezoelectric film technology—a core innovation from this forward-thinking MedTech start-up.
From start-up to Novartis
Vivacta, a successful start-up born out of DTSL, made significant strides in the healthcare industry. Through the collaborative efforts of Maddison and ITL, they designed and constructed over 100 pilot units, garnering valuable evidence. This remarkable achievement caught the attention of healthcare giant Novartis, leading to Vivacta's acquisition for an impressive £90m in 2012. In 2016, Nji reached another milestone by obtaining CE marking, allowing the system to be legally sold in the European Union and all other countries acknowledging the CE Mark.
Cartridge and sampling usability
Our team leveraged both physical and digital prototypes to conduct usability studies with Vivica and prospective users, aiming to enhance and authenticate the design. Overcoming distinctive challenges, we focused on optimizing the usability of the cartridge. Our primary goals were to make it intuitive, user-friendly, and safe. Additionally, we prioritized ensuring that users did not obstruct the optical windows with finger marks, which, are essential for viewing the sample.

User testing and refinement
During the initial development phase, the device prototypes underwent thorough user testing. This iterative process allowed for continuous enhancements, resulting in a robust usability technical file. The human factors submission showcased Maddison’s dedication to meeting industry standards. Our methodical approach to building and testing prototypes underscored the value of our user-centred design methods. Consequently, the final design emerged as user-friendly and intuitive, increased the likelihood of adoption among clinicians and physicians who now rely on the device.