Helping a Start-up get to market
The Phagenesis team set out to improve the outcomes of patients suffering from swallowing difficulties caused by strokes, the revolutionary treatment uses neural stimulation at the patient's pharynx with a catheter, promoting neural plasticity to aid in recovery. Maddison was selected for our expertise in industrial design, mechanical design and user interface development. Our essential skills where key for adapting Phagenesis into a practical product suitable for hospitals, clinics, and care homes. The main challenge was translating the complex research into a user-friendly solutions for everyday use.

Phagenesis wins Bionow Award for Healthcare Project of the Year
The critical design challenge
The Phagenesis solution faced several challenges, the product had to be simple to use, easy to clean, robust, cost effective and easy to manufacture. The industrial design needed to communicate a high-quality aesthetic to support the Phagenesis emerging brand. Maddison were brought in at the early design stage to drive the mechanical and industrial design aspects of the product development for base station and catheter.
As well as meeting the functional requirements, the Phagenesis Dysphagia Treatment System had to conform to the relevant standards including IEC 60601 (electrical medical systems), 62366 (usability) and 10993 (biocompatibility.) Maddison worked with their partners Tactiq who designed the software and electronics.
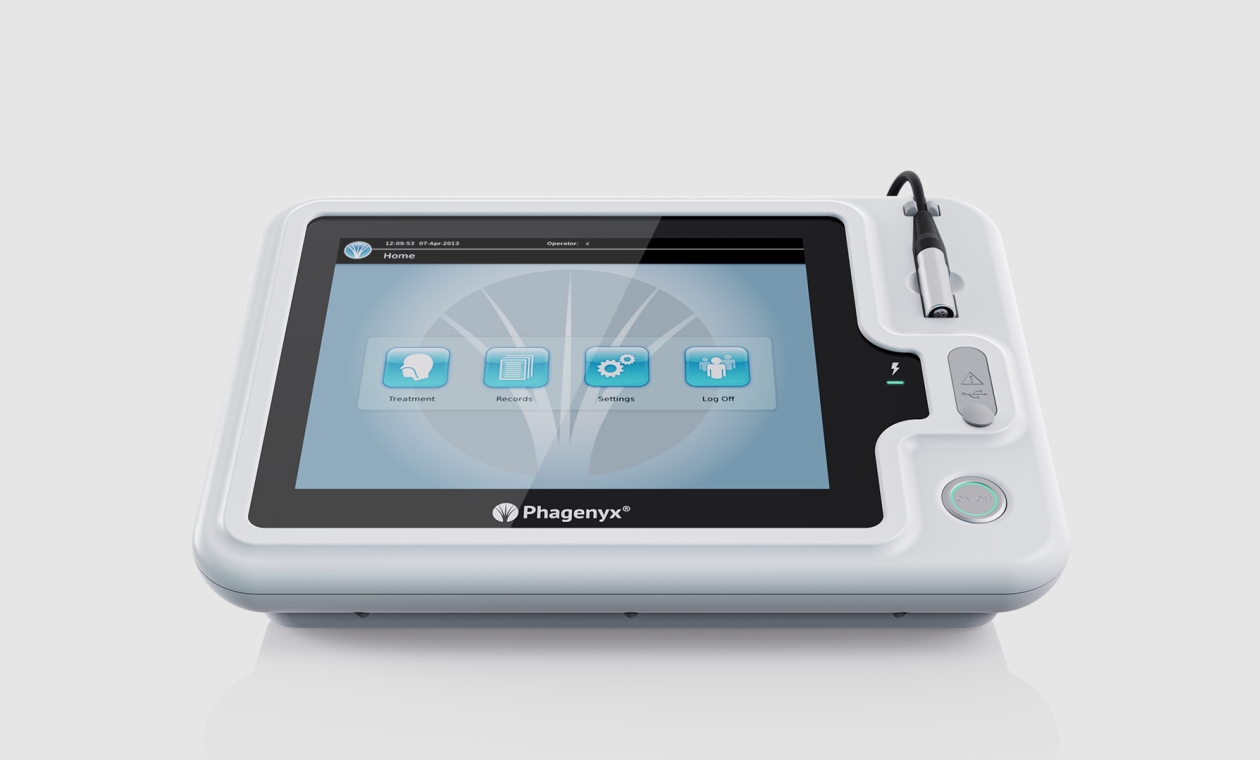
UI Development
Critical to market and clinical adoption the team focused on creating a user-friendly Graphical User Interface (GUI). This intuitive GUI was designed to be easily mastered by both experienced users and novice Speech and Language Therapists, promoting efficient learning. As a certified medical device by the FDA and EU, our GUI and human-machine interface adhere strictly to the ISO 62366 Human Factors guidelines during the development process. Read more about GUI development...
From concept to production
Maddison played a role in the entire design process, covering early stages, production, and post-production support. We collaborated closely with manufacturing partners to bring the final product to market.
Phagenesis achieved a notable milestone with the CE mark granted in 2012 for dysphagia treatment. Additionally, the Phagenyx® System received FDA Breakthrough Device Designation in January 2020.