40 years of experience
We are a distinguished product development company with 40 years of experience.
- Our seasoned team comprises dedicated, problem-solving engineers and designers, well-equipped to tackle challenging projects.
- Proven track record in successfully developing medical, scientific, industrial, and consumer products.
- Comprehensive range of services tailored to medical device development.
- Turnkey product development service, collaborating with a network of partner companies specializing in software and electronics.
- Organized for the delivery of world-class design and development at highly competitive prices.
We are organised to be flexible and cost efficient
Maddison has an efficient and innovative organizational structure which offers unique advantages over traditional consultancy models.
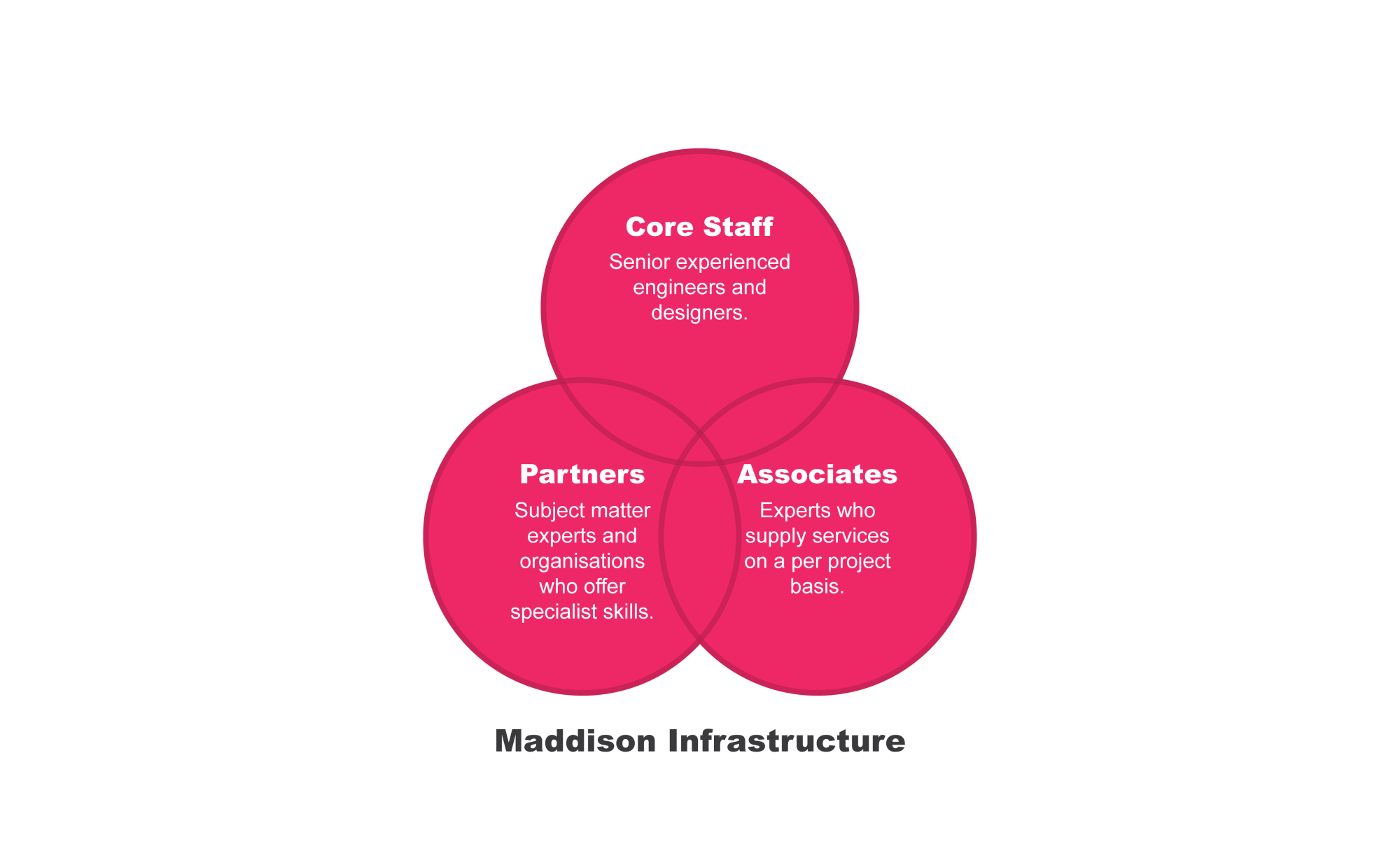
Maddison's organizational structure provides distinct benefits
- Expertise: We assign dedicated experts to your project, ensuring that only the most qualified professionals work on your project. Unlike the traditional model, we prioritize specialized knowledge and skills over simply utilizing available staff.
- Cost-effectiveness: With Maddison, you only pay for the experts you need. Unlike the traditional model that includes overhead costs for staff and equipment you may not utilize, our approach focuses on optimizing your budget by aligning it with the specific requirements of your project.
- Speed: When you choose Maddison, you gain access to a dedicated and experienced team tailored to your needs. We prioritize prompt and efficient delivery, with a focus on meeting your project requirements.
To support this system, we have made significant investments in management systems and cloud infrastructure. These investments enable us to quickly assemble high-performing teams and seamlessly integrate expertise as required. For more information on our approach, you can read the article below.
Download article on the technology enabling Maddison's unique structure

David founded Maddison in 1982 and now serves as the Chairman, providing valuable direction, guidance, and strategic vision. Throughout his career, he has been actively involved in supporting UK design and technology, particularly in helping universities and spin-outs commercialize their intellectual property. His goal is to enhance the value of technologies and leverage design tools to speed up spinning out or licensing intellectual property.
In December 2006, David was appointed as a mentor to ISIS (now Set Squared) and has collaborated closely with the Design Council's "Designing Demand" program, offering mentorship to their technology transfer team. In recognition of his expertise, he assumed the role of Designer-in-Residence at the University of Manchester in 2010, focusing on facilitating the spin-out of scientific and medical technologies.
Under David's leadership, Maddison has forged strong technology transfer relationships with renowned UK universities, including Cambridge, Imperial, UCL, Oxford, Manchester, and Leeds, resulting in the successful spin-out of multiple acclaimed medical devices.

Bringing his expertise as the former Design Director at PA Consulting, Rob now leads Maddison as the Managing Director. With a rich background in working with prominent industry leaders like GE and Fitch, Rob has played a key role in designing and developing a wide range of medical devices. His profound understanding of mitigating risks in product development and navigating regulatory requirements for the commercialization of medical technologies has been instrumental in his successful collaborations.
Throughout his career, Rob has partnered with esteemed clients including Sanofi, J&J, Roche, GE Ethicon, Bausch Lomb, and Merck, contributing to the advancement of innovative medical device solutions.
Nick is a highly experienced senior design engineer at Maddison, bringing over 20 years of invaluable expertise to the team. Throughout his tenure, Nick has been involved in the design and technical development of a diverse range of products, including medical devices, life sciences and scientific equipment, industrial and consumer goods. His exceptional problem-solving skills and ability to adapt swiftly to new project requirements have established him as a key team member, leading numerous projects for esteemed clients such as P&G, Vivacta, Novartis, Phagenesis, and Creavo.
With a keen eye for detail and extensive knowledge of mechanical processes and material selection, Nick consistently delivers designs that exceed expectations. His profound understanding of product design plays a vital role in contributing to Maddison's Quality System, ensuring that every project meets the highest standards of excellence.

Ben is the design directors at Maddison and he brings a unique blend of scientific expertise and product development experience to the team. With a proven track record, Ben has spearheaded the successful development of ground-breaking products like the Sure-pulse neonatal monitor and the M-mark stroke therapy system. From the initial concept to achieving accreditation and CE marking, Ben's leadership has guided these innovations to become commercially viable products.
Beyond his accomplishments in product development, Ben has also garnered extensive experience in working with start-ups and spin-outs, adeptly maximizing budgets to bring their products to market. He possesses a deep understanding of the regulatory requirements associated with developing medical devices. Whilst, having the skills to invent practical mechanical and digital products that are not only intuitive and attractive but also cost-effective to manufacture.

Oliver is Maddison’s Design Director, and has more than 30 years’ experience designing products of all kinds, including many medical products spanning optics-based devices, microfluidics, drug delivery, point of care, and more. Although an industrial designer by training, Oliver has extensive knowledge and experience in the engineering of products, both technical problem solving and manufacturing. His sensitive and insightful understanding of user needs combined with an ability to create iconic, award-winning designs brings powerful product design skills to the team, and has provided the backbone to may successful projects at Maddison. Oliver has been involved with projects for the likes of GSK, P&G, Alere, RB, Vivacta, and a number of successful University and spin-out companies such as Phagenesis and Creavo.

Jonathan has more than 10 years of experience in the product design industry, specializing in user-centred design, interface design, user research and human factors. Throughout his career, he has contributed to large range of product development projects. Currently, he leads a drug delivery project for a prominent funding organization. Jonathan excels in both industrial design and user interface (UI) and user experience (UX) design, ensuring seamless user experiences across physical and digital products.

Gill serves as the accounts manager at Maddison, bringing valuable experience to the team. With a four-year tenure at Maddison. Gill plays a vital role in overseeing accounting functions, supplier management, and day-to-day operations. This includes the challenging task of managing and controlling the design team. Gill's meticulous attention to detail and strategic approach contribute to effective financial management and smooth operations at Maddison.

Paul and Maddison have been collaborating for more than 15 years, forming a strong partnership. Paul, an experienced engineer and innovative thinker, brings his expertise in developing scientific and medical devices. His creative approach and deep knowledge allow him to contribute beyond electronics, including areas like microfluidics, sensing, and optics. Together, Paul and Maddison strive to provide outstanding solutions that meet the highest standards of quality and innovation.

Andrew served as the technical director at Maddison from 2003 to 2014. Since 2014, he has been working with Maddison on a per-project basis. Andrew is a hands-on design engineer with a diverse background in various technologies. He applies his extensive knowledge and skills to each project as needed. Andrew is involved in multiple stages of the project, including project management, engineering concept generation, prototype development and production transition. He has played a leading role in the development of a large range of medical and life science products.

Inspired Usability is lead by Miranda Newbury.
At Inspired Usability, we support the medical device and pharmaceutical industry by helping to create outstanding products that are accessible, user friendly and above all, safe.
Whether you are starting your medical device or combination product journey and want true user insight, or you are navigating the Human Factors regulatory requirements, Inspired Usability can provide you with design support and services tailored to your individual project needs. We excel in applying independent usability testing that goes beyond the standard regulatory Human Factors requirements to get to the heart of what makes a great product. Inspired Usability Limited has been accredited by the Chartered Institute of Ergonomics & Human Factors (CIEHF.)

Dr Phil Marsden formed Unitive Design & Analysis to bring high end science in imaging and sensing systems to leading edge medical and industrial organisations. He is a long-term member of the IOP and the IEEE and sits on several committees and advisory boards, including the IOP Medical Physics Group.
Unitive and Maddison have a synergistic set of skills and knowhow, so are able to create teams uniquely qualified to co-develop breakthrough medical and in-vitro devices which require complex interactions between hardware and software.
A trusted partner, Unitive's highly skilled multi-disciplinary team enables us to bring unique benefits to our clients, leveraging their experience in design concept and early feasibility to pilot manufacturing and support for regulatory submission.

James Yorkshades and Tristan Hughes are the founders of Blue Lightning Solutions (BLS). BLS is a team of skilled and experienced electronics consultants specializing in productization helping clients to manufacture, improve and distribute their products.
With a wide range of industry expertise, including scientific equipment, medical devices, Invitro Diagnostic devices, consumer goods, and challenging environments, BLS offers technology and electronics consulting services to support a variety of projects. Their goal is to deliver exceptional results and ensure the success of each endeavour.