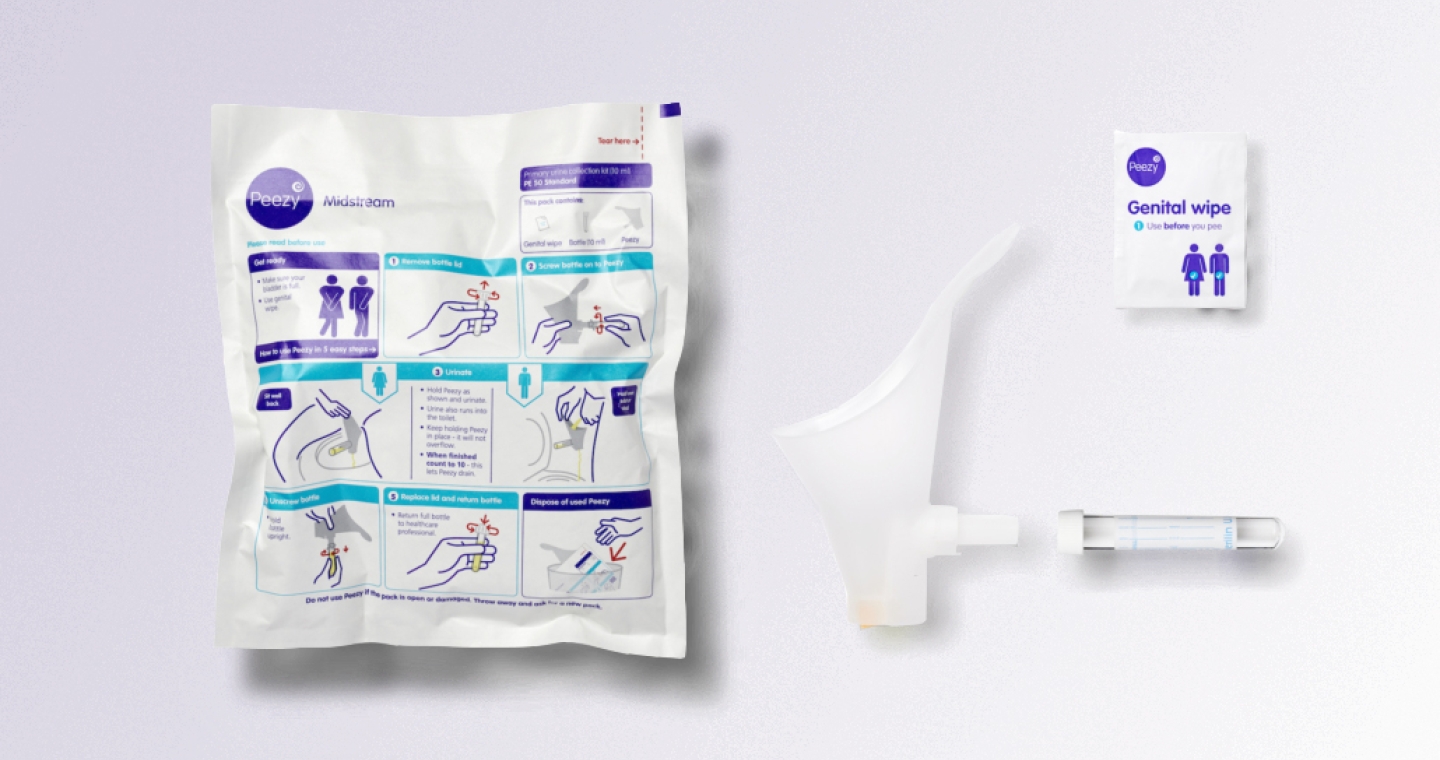
Instructions for use
Instructions for Use (IFU's) are sometimes overlooked in the product development process, but their importance should not be underestimated. Developing intuitive and straightforward IFU's is a valuable skill that can bring benefits in terms of time, cost, and complexity savings.
At Maddison we understand the significance of well-crafted IFU's. Our experienced team can assist you in developing user-friendly IFU's that meet regulatory standards. By focusing on simplicity, clarity, and conciseness, we ensure that users can easily understand and operate your device.
At Maddison we have experience in:
- Developing graphic, interactive and video IFU’s
- Developing secondary packaging, with integrated infographics and labelling
- Conducting trials to develop, test and refine (formative) IFU's
We understand the standards we need to work with:
- ISO 13485 Medical devices - Quality management systems
- ISO 20417 Medical devices - Information to be supplied by the manufacture
- ISO 15223 Medical devices - Symbols to be used with information to be supplied by the manufacturer